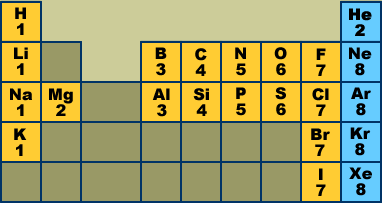
In chemistry and physics, a valence electron is an outer shell electron that is associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. Valence Electrons of all the elements in the Periodic Table Refer to graph, table and property element trend below for Valence Electrons of all the elements in the periodic table. We have shown the Valence Electrons of the elements for which reliable data is available. Valence Electrons Graph - Valence Electrons of all the elements in graph.
- Apr 20, 2021 Now, for the oxygen atom, its atomic number is eight whereas the electronic configuration is 1s2 2s2 2p4. As p shell can allow six electrons, there is a scarcity of only two electrons. Due to this, the maximum valence electrons in a single oxygen atom is six.
- .For atoms with LESS than 4valence electrons, they’re going to lose/give upelectrons to form positive cations.For atoms with MORE than 4valence electrons, they’re going to gain/stealelectrons to form negative anions.For atoms with 4 valence electrons, it can go either way.For atoms with 8 valence electrons, there is no change.
Ionic Bonding
Soduim atom'>We have learned that atoms tend to react in ways that create a full valence shell, but what does this mean? Sodium (Na), for example, has one electron in its valence shell. This is an unstable state because that valence shell needs eight electrons to be full. In order to fill its valence shell, sodium has two options:
- Find a way to add seven electrons to its valence shell, or
- Give up that one electron so the next lower energy shell (already full) could become its new valence shell.
Which do you think is more easily accomplished?
Right, giving up one electron! Atoms like sodium, with only one or two electrons in a valence shell that needs eight electrons, are most likely to give up their valence electrons to achieve a stable state. All these atoms need is another atom that can attract their electrons!
An atom such as chlorine (Cl), that contains seven electrons in its valence shell, needs one more electron to have a full valence shell. If we use the same logic as we did for sodium, we should conclude that chlorine would rather gain one more electron than lose all seven of its valence electrons to achieve stability. Under the right conditions, atoms like chlorine will steal an electron from nearby atoms like sodium. This ability to pull electrons away from other atoms is termed electronegativity. Atoms with a valence shell that is almost full are more likely to be electronegative because they have greater reason to pull electrons towards them. Electronegative atoms are not negatively charged, but they are more likely to become negatively charged.
Bahan evo pet seed. When an electron moves from one atom to another, both atoms become ions. An ion is any atom that has gained electrons to have a negative charge (anion) or lost electrons to have a positive charge (cation). An easy way to remember that a cation has a positive, or +, charge is to think of the letter t in the word cation as a + sign.
Once an atom becomes an ion, it has an electrical charge. Ions of opposite charge are attracted to one another, forming a chemical bond, an association formed by attraction between two atoms. This type of chemical bond is called an ionic bond because the bond formed between two ions of opposite charge. The sodium cation (Na+) and the chlorine anion (Cl-) are attracted to one another to form sodium chloride, or table salt.
Although ionic bonds are very strong, they can be relatively easily broken if another attractive ion (or polar molecule) comes around. An ionic bond is formed when two ions of opposite charge come together by attraction, NOT when an electron is transferred.
Think of ionic bond formation as the second step in a two step process:
- Two atoms each become ions. The atoms could have become ions in previous reactions with other atoms or the atoms may have reacted with each other, transferring the electron(s) from one to the other.
- The two ions of opposite charge “see” one another and become attracted enough to form the bond.
Ionic Bonding
This video visually illustrates how atoms form ionic bonds.
Create an Ionic Bond
In this activity, you'll create cations and anions and watch the ionic bond form.
Covalent Bonding
Carbon atom'>In ionic bonding, we looked at atoms with either one or two electrons in their valence shell and atoms that only needed one or two electrons to fill their valence shell. What happens when an atom, carbon (C) for example, has four valence electrons? Carbon would need to either lose four electrons or gain four electrons in order to have a full valence shell. Both of these situations would cause carbon to have a very strong charge, which would probably make it as unstable as having an incomplete valence shell! For atoms like carbon, there is another option: sharing.
When two atoms each need additional electrons to fill their valence shells, but neither is electronegative enough to steal electrons from the other, they can form another kind of chemical bond called a covalent bond. In covalent bonds, two atoms move close enough to share some electrons. The electrons from each atom shift to spend time moving around both atomic nuclei.
In the most common form of covalent bond, a single covalent bond, two electrons are shared, one from each atom’s valence shell. Double covalent bonds where four electrons are shared, and triple covalent bonds where six electrons are shared, are also commonly found in nature.
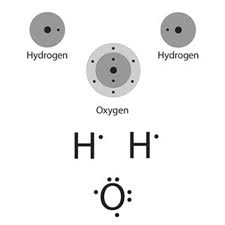
How do we know if and when covalent bonds will form? Atoms will form as many covalent bonds as it takes to fill their valence shell. This means that carbon, our previous example, will need to form four covalent bonds in order to fill its outermost shell. In each of the four bonds, carbon will contribute one electron and the other atom will contribute one electron, supplying carbon with eight electrons effectively orbiting its nucleus. Atoms like oxygen (O) will form two covalent bonds because they already have six valence electrons and only need two more electrons obtained by sharing. Put another way, oxygen shares two of its six valence electrons in covalent bonds while keeping four valence electrons for itself (4 unshared oxygen electrons + 2 shared oxygen electrons + 2 shared electrons from the other atoms = 8 total electrons).
In shell models, the shared electrons are shown within the overlapping region of the valence shells to represent the fact they are shared, but the electrons are actually moving around both nuclei and could be found anywhere around either nucleus at a given time.
For simplicity, we often draw covalent bonds as straight lines between atoms to represent the structural formula. Each line represents a single covalent bond (two shared electrons), so double lines represent a double covalent bond (four shared electrons).
Covalent bonds are usually found in atoms that have at least two, and usually less than seven, electrons in their outermost energy shell, but this is NOT a hard and fast rule. Hydrogen, for example, is a unique atom that bears closer examination. Because hydrogen only has one electron surrounding its nucleus, its valence shell is the first energy shell, which only needs two electrons to be full. Hydrogen tends to form covalent bonds because a single covalent bond will fill its shell. However, the one-proton nucleus is very weak and has trouble keeping the shared electrons around the hydrogen atom for very long.
Covalent Bonding
This video visually illustrates how atoms form covalent bonds.


Lithium
Other Kinds of Bonding
When a covalent bond forms between atoms of similar electronegativity, the shared electrons tend to spend equal time around each nucleus. What happens if a bond is formed between atoms like oxygen, which is highly electronegative, and hydrogen, which is not? The oxygen atom tends to pull the shared electrons over to its side of the bond more often than the hydrogen atom does, resulting in polarity, or a partial separation of charge between atoms. The electrons don’t actually leave the less electronegative atom, but they do spend less time on that side. This results in two poles, one slightly positive and one slightly negative. This is called a polar bond, while covalent bonds where electrons are shared equally are called nonpolar.
Element O Valence Electrons

Polar covalent bonds are the source of an additional kind of association called a hydrogen bond. In a hydrogen bond, the partially positive end of a polar covalent molecule is attracted to the partially negative end of another polar covalent molecule. For example, water is composed of two hydrogen atoms covalently bonded to a single oxygen atom. In a container full of water molecules, the hydrogen atoms of each water molecule are attracted to the oxygen atoms of other water molecules, forming hydrogen bonds between all of the molecules in the container of water. Hydrogen bonds are weak compared to covalent bonds, but they are strong enough to affect the behavior of the atoms involved. This leads to many important chemical properties in water and other molecules.
